Efir
Kimyoda efer oksokislotadan olingan birikma boʻlib, unda kamida bitta gidroksil guruhi (−OH) yoki alkoksi guruhi bilan almashtiriladi (−O−), karbosiklik kislota va spirtni almashtirish reaksiyasida boʻlgani kabi, Glitseridlar-glitserinning yog' kislota eferlari bo'lib, ular biologiyada muhim ahamiyatga ega. lipidlarning asosiy sinfiga hayvonlarning yogʻlari va o'simlik moylarining asosiy qismi hisoblanadi.
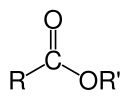
Eferlar odatda yoqimli hidga ega, past molekulyar ogʻirlikdagilari odatda xushboʻy moddalar sifatida ishlatiladi va efir moylari feromonlarda mavjud. Ular keng koʻlamli plastmassalar, plastifikatorlar, qatronlar va laklar uchun yuqori sifatli erituvchilar sifatida ishlaydi[1]. Tijorat bozorida sintetik moylash materiallarining eng katta sinflaridan biri hisoblanadi[2]. Polyesterlar muhim plastik boʻlib, monomerlarni efer qismlari bilan bogʻlangan. Fosfoeferlar DNK molekulalarining asosini tashkil qiladi. Nitrogliserin kabi nitrat eferlari portlovchi xususiyatlari bilan mashhur.
Nomenklaturasi
tahrirEtimologiya
tahrirEfer soʻzini 1848-yilda nemis kimyogari Leopold Gmelin[3], nemis Essigäther qisqarishi sifatida „sirka efirini“ ishlab chiqqan.
IUPAC nomenklaturasi
tahrirEferlarning nomlari spirt va asosiy kislotadan olingan boʻlib, ikkinchisi organik yoki noorganik boʻlishi mumkin. Eng oddiy karboksilik kislotalardan olingan efirlar odatda anʼanaviy, „ arzimas nomlar“ boʻyicha nomlanadi. Masalan format, atsetat, propionat va butirat, IUPAC nomenklaturasi metanoat, etanoat, propanoat va butanoatdan farqli oʻlaroq. Boshqa tomondan, murakkabroq karboksilik kislotalardan olingan esterlar, aksincha, kislota nomidan keyin qoʻshilgan IUPAC nomidan foydalangan holda koʻproq nomlanadi. Masalan, heksil kaprilat nomi bilan ham tanilgan efer oktanoat formulasi quyidagicha CH3(CH2)6CO2(CH2)5CH3.
Organik eferlarning kimyoviy formulasi odatda RCO2R’ shaklni oladi, bu yerda R va R' mos ravishda karbosilik kislota va spirtning uglevodorod qismlari. Masalan, butanol va sirka kislotasidan (sistematik ravishda etanoik kislota) olingan butil asetat (tizimli butil etanoat) CH3CO2C4H9. Muqobil taqdimotlarda keng tarqalgani CH3COOC4H9.
Siklik efirlar organik yoki noorganik kislotadan olinganligidan qatʼi nazar, laktonlar deb ataladi. Organik laktonga misollardan biri γ-valerolaktondir.
Ortoeferlari
tahrirOrganik efirlarning kam uchraydigan sinfi ortoesterlardir, quyidagichaRC(OR′)3 formulaga ega. Trietilortoformat (HC(OC2H5)3) nomi boʻyicha (lekin sintezi emas) ortoform kislota (HC(OH)3) va etanol.
Noorganik efirlar
tahrirEferlarni noorganik kislotalardan ham olish mumkin.
- Fosfor kislotasi fosfat efirlarini hosil qiladi, masalan, trifenilfosfat
- sulfat kislota sulfat efirlarini hosil qiladi, masalan, dimetilsulfat
- nitrat kislota nitrat efirlarini hosil qiladi, masalan, metil nitrat
- borik kislotasi boratlar hosil qiladi, masalan, trimetilborat
- karbonat kislota karbonat efirlarini hosil qiladi, masalan, etilen karbonat
Tautomerlar sifatida mavjud boʻlgan noorganik kislota turli xil efirlarni hosil qiladi.
- fosfor kislotasi ikki xil fosfit efirlarini hosil qiladi, masalan, trietilfosfit (P(OEt)3) va dietilfosfit (HP(O)(OEt)2).
Noorganik kislotalar barqaror boʻlmagan yoki qiyin efirlarni hosil qiladi.
- hech qachon aniqlanmagan xrom kislotasi di-tert-butilxromat hosil qiladi
- kam uchraydigan oltingugurt kislotasi dimetilsulfitni hosil qiladi
Asosan yuzlab maʼlum boʻlgan barcha metall va metalloid alkoksidlarni, gipotetik kislotalarning efirlari deb tasniflash mumkin.
Tuzilishi va bogʻlanishi
tahrirEferlar karbonil markazini oʻz ichiga oladi, bu 120° C–C–O va O–C–O burchaklarini hosil qiladi. Amidlardan farqli oʻlaroq, efirlar strukturaviy moslashuvchan funktsional guruhlardir, chunki C–O–C aloqalari atrofida aylanishi past toʻsiqga ega. Ularning moslashuvchanligi va past polaritesi ularning jismoniy xususiyatlarida namoyon boʻladi. Ular mos keladigan amidlarga qaraganda kamroq qattiq (pastki erish nuqtasi) va koʻproq uchuvchan (pastki qaynash nuqtasi) boʻlishga moyil[4]. Efirlardagi alfa-vodorodlarning pKa 25 ga teng[5].
Koʻpgina efirlar konformatsion izomeriya uchun potentsialga ega, ammo ular giperkontugatsiya va dipolni minimallashtirish effektlari kombinatsiyasi tufayli s-trans (yoki E) muqobilidan koʻra s-cis (yoki Z) konformatsiyasini qabul qilishga moyildirlar. Z konformatsiyasini afzal koʻrishga, agar mavjud boʻlsa, oʻrinbosar va erituvchining tabiati taʼsir qiladi[6][7]. Kichik halqali laktonlar s-trans siklik tuzilishi tufayli konformatsiyalanadi.
Fizik xususiyatlari va xarakteristikasi
tahrirEferlar qutbli, ammo spirtlarga qaraganda kamroq qutblidir. Ular vodorod bog'lanishlarida vodorod bog'larini qabul qiluvchi sifatida ishtirok etadilar, lekin spirtlardan farqli oʻlaroq, vodorod bogʻlari donorlari sifatida harakat qila olmaydilar. Vodorod bogʻlanishida ishtirok etish qobiliyati suvda bir oz eruvchanlikni beradi. Vodorod bogʻlarini berish qobiliyati yoʻqligi sababli, efirlar oʻz-oʻzidan birlashmaydi. Shunday qilib, eferlarga oʻxshash molekulyar ogʻirlikdagi karbosilik kislotalarga qaraganda ancha uchuvchan[4].
Xarakterlash va tahlil qilish
tahrirEferlar odatda gaz xromatografiyasi yordamida ularning uchuvchanligidan foydalangan holda aniqlanadi. Eferlar uchun IQ spektrlari 1730–1750 sm−1 oraligʻida kuchli oʻtkir chiziqqa va vC=O ga tayinlangan. Bu choʻqqi karbonilga biriktirilgan funksional guruhlarga qarab oʻzgaradi. Misol uchun, benzol halqasi yoki karbonil bilan konjugatsiyadagi qoʻsh bogʻlanish toʻlqin sonini taxminan 30 sm−1 ga tushiradi.
Ilovalar va hodisalari
tahrirEferlar tabiatda keng tarqalgan va sanoatda keng qoʻllanadi. Tabiatda yog'lar, glitserin va yog' kislotalaridan olingan eferlar trieferlardir[9]. Eferlar olma, nok, banan, ananas va qulupnay kabi mevalarning xushboʻy hidlariga ega[10]. Har yili sanoatda bir necha milliard kilogramm poliefer ishlab chiqariladi, muhim mahsulotlari sifatida polietilen tereftalat, akrilat efir va sellyuloza asetatlar kiradi.
Triglitseridning vakili zigʻir yogʻida, linoleik kislotadan (pastki oʻngda), <span id="mw7A" style="color:red;"><b id="mw7Q">alfa-linolenik kislotadan</b></span> (chapda) va oleyk kislotadan (oʻng tomonda) olingan triester (triglitserid) mavjud.
Tayyorgarlik
tahrirEferifikatsiya kimyoviy reaksiyaning umumiy nomi boʻlib, unda ikkita reaktiv (odatda spirt va kislota) reaksiya mahsuloti sifatida efir hosil qiladi. Eferlar organik kimyo va biologik materiallarda keng tarqalgan boʻlib, koʻpincha yoqimli xarakterli meva hidiga ega. Bu ularning xushbo'y hid va lazzat sanoatida keng qoʻllanishiga olib keladi. Efer aloqalari koʻplab polimerlarda ham mavjud.
Karbosilik kislotalarning spirtlar bilan eferlanishi
tahrirKlassik sintez Fisher eferifikatsiyasi boʻlib, u karbosiklik kislotani alkogol bilan suvsizlantiruvchi vosita ishtirokida davolashni oʻz ichiga oladi.
- <math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow class="MJX-TeXAtom-ORD"><mstyle displaystyle="true" scriptlevel="0"><mrow class="MJX-TeXAtom-ORD"><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH <=> RCO2R' + H2O}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH <=> RCO2R' + H2O}} </mn></mrow></msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH <=> RCO2R' + H2O}} </mtext><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH <=> RCO2R' + H2O}} </mo><msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH <=> RCO2R' + H2O}} </mtext><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH <=> RCO2R' + H2O}} </mo></msup><mtext> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2H + R’OH <=> RCO2R' + H2O}} </mtext><mrow class="MJX-TeXAtom-REL"><mover><mrow class="MJX-TeXAtom-OP MJX-fixedlimits"><mrow class="MJX-TeXAtom-ORD"><mpadded depth="0" height="0"><mrow class="MJX-TeXAtom-ORD"><mo stretchy="false">Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH <=> RCO2R' + H2O}} </mo></mrow><mrow class="MJX-TeXAtom-ORD"><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH <=> RCO2R' + H2O}} </mo></mrow></mpadded></mrow></mrow><mrow class="MJX-TeXAtom-ORD"><mstyle displaystyle="false" scriptlevel="0"><mrow class="MJX-TeXAtom-ORD"><mrow class="MJX-TeXAtom-ORD"><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH <=> RCO2R' + H2O}} </mo></mrow><mrow class="MJX-TeXAtom-ORD"><mo stretchy="false"> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2H + R’OH <=> RCO2R' + H2O}} </mo></mrow></mrow></mstyle></mrow></mover></mrow><msubsup><mtext> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2H + R’OH <=> RCO2R' + H2O}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH <=> RCO2R' + H2O}} </mn></mrow></msubsup><msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH <=> RCO2R' + H2O}} </mtext><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH <=> RCO2R' + H2O}} </mo></msup><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH <=> RCO2R' + H2O}} </mo><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH <=> RCO2R' + H2O}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2H + R’OH <=> RCO2R' + H2O}} </mn></mrow></msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH <=> RCO2R' + H2O}} </mtext></mrow></mstyle></mrow><annotation encoding="application/x-tex"> </annotation></semantics></math>Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH <=> RCO2R' + H2O}}
Bunday reaksiyalar uchun muvozanat konstantasi, masalan, etil asetat uchun taxminan 5 ga teng[11]. Katalizator boʻlmaganda reaksiya sekin boradi. Sulfat kislota bu reaksiyaning tipik katalizatoridir. Polimer sulfonik kislotalar kabi koʻplab boshqa kislotalar ham qoʻllanadi. Eferifikatsiyaning qaytarilishi yuqori boʻlgani sababli, eferning rentabelligini Le Chatelier prinsipi yordamida yaxshilash mumkin.
- Spirtli ichimliklarni koʻp miqdorda ishlatish (yaʼni, erituvchi sifatida).
- Suvsizlantiruvchi vositadan foydalanish. Sulfat kislota nafaqat reaksiyani katalizlaydi, balki suvni (reaksiya mahsuloti) sekvestr qiladi. Molekulyar elaklar kabi boshqa quritish vositalari ham samarali.
- Dean-Stark apparati bilan birgalikda toluol bilan kam qaynaydigan azeotroplar sifatida distillash kabi jismoniy vositalar bilan suvni olib tashlash.
Spirtli ichimliklar va karbosiklik kislotalar aralashmalarining suvsizlanishiga olib keladigan reaktivlar sifatida maʼlum. Steglich eferifikatsiyasi, bu yumshoq sharoitda eferlarni hosil qilish usulidir. Bu usul peptid sintezida mashhur boʻlib, substratlar yuqori issiqlik kabi ogʻir sharoitlarga ega. DCC (disikloheksilkarbodiimid) karbosiklik kislotani keyingi reaksiyaga faollashtirish uchun ishlatiladi. 4-Dimetilaminopiridin (DMAP) asil-transfer katalizatori sifatida ishlatiladi.
Spirtli ichimliklar va karbosiklik kislotalar aralashmalarini suvsizlantirishning yana bir usuli Mitsunobu reaksiyasi.
- <math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow class="MJX-TeXAtom-ORD"><mstyle displaystyle="true" scriptlevel="0"><mrow class="MJX-TeXAtom-ORD"><msubsup><mtext> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mn></mrow></msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mtext><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mo><msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mtext><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mo></msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mtext><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mo><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mtext><msubsup><mrow class="MJX-TeXAtom-ORD"><mo stretchy="false"> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mo><msubsup><mtext> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mn></mrow></msubsup><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mn></mrow></msubsup><mo stretchy="false"> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mo></mrow><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mn></mrow></msubsup><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mo><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mn></mrow></msubsup><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mn></mrow></msubsup><mo stretchy="false"> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mo><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mn></mrow></msubsup><msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mtext><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mo></msup><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mo><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mtext><msubsup><mrow class="MJX-TeXAtom-ORD"><mo stretchy="false"> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mo><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mn></mrow></msubsup><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mn></mrow></msubsup><mo stretchy="false"> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mo></mrow><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mn></mrow></msubsup><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mo><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mn></mrow></msubsup><msubsup><mtext> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mn></mrow></msubsup><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}} </mn></mrow></msubsup></mrow></mstyle></mrow></semantics></math>Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2H + R’OH + P(C6H5)3 + R2N2 -> RCO2R' + OP(C6H5)3 + R2N2H2}}
Karbosiklik kislotalarni diazometan yordamida eferlash mumkin.
- <math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow class="MJX-TeXAtom-ORD"><mstyle displaystyle="true" scriptlevel="0"><mrow class="MJX-TeXAtom-ORD"><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mtext> </mtext><mo> </mo><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mo stretchy="false"> </mo><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mo> </mo><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup></mrow></mstyle></mrow></semantics></math>
Ushbu diazometandan foydalanib, karboksilik kislotalarning aralashmalarini gaz xromatografiyasi orqali tahlil qilish uchun, miqdoriy rentabelliklarda ularning metil efirlariga aylantirilish mumkin. Ixtisoslashtirilgan organik sintetik operatsiyalarda foydalidir, ammo keng koʻlamli ilovalar uchun juda xavfli va qimmat hisoblanadi.
Karboksilik kislotalarning epoksidlar bilan eferifikatsiyasi
tahrirKarboksilik kislotalar epoksidlar bilan ishlov berish orqali eferlanadi va β-gidroksiefirlarni beradi.
- <math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow class="MJX-TeXAtom-ORD"><mstyle displaystyle="true" scriptlevel="0"><mrow class="MJX-TeXAtom-ORD"><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mtext> </mtext><mo> </mo><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mtext> </mtext><mo stretchy="false"> </mo><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mo stretchy="false"> </mo><mtext> </mtext><mo stretchy="false"> </mo></mrow><mtext> </mtext></mrow></mstyle></mrow><annotation encoding="application/x-tex"> </annotation></semantics></math>
Ushbu reaksiyada akril kislotadan, vinil efer qatronlar ishlab chiqarishda qoʻllanadi.
Asilxloridlar va kislotali angidridlarning alkogollizi
tahrirSpirtli ichimliklar asilxloridlar va kislota angidridlari bilan reaksiyaga kirishib, efirlarni hosil qiladi.
- <math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow class="MJX-TeXAtom-ORD"><mstyle displaystyle="true" scriptlevel="0"><mrow class="MJX-TeXAtom-ORD"><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCOCl + R’OH -> RCO2R' + HCl}} </mtext><mo> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCOCl + R’OH -> RCO2R' + HCl}} </mo><msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCOCl + R’OH -> RCO2R' + HCl}} </mtext><mo> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCOCl + R’OH -> RCO2R' + HCl}} </mo></msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCOCl + R’OH -> RCO2R' + HCl}} </mtext><mo stretchy="false"> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCOCl + R’OH -> RCO2R' + HCl}} </mo><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCOCl + R’OH -> RCO2R' + HCl}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCOCl + R’OH -> RCO2R' + HCl}} </mn></mrow></msubsup><msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCOCl + R’OH -> RCO2R' + HCl}} </mtext><mo> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCOCl + R’OH -> RCO2R' + HCl}} </mo></msup><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCOCl + R’OH -> RCO2R' + HCl}} </mo><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCOCl + R’OH -> RCO2R' + HCl}} </mtext></mrow></mstyle></mrow><annotation encoding="application/x-tex"> </annotation></semantics></math>Failed to parse (sintaktik xato): {\displaystyle \ce{RCOCl + R’OH -> RCO2R' + HCl}}
- <math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow class="MJX-TeXAtom-ORD"><mstyle displaystyle="true" scriptlevel="0"><mrow class="MJX-TeXAtom-ORD"><msubsup><mrow class="MJX-TeXAtom-ORD"><mo stretchy="false"> Failed to parse (sintaktik xato): {\displaystyle \ce{(RCO)2O + R’OH -> RCO2R' + RCO2H}} </mo><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{(RCO)2O + R’OH -> RCO2R' + RCO2H}} </mtext><mo stretchy="false"> Failed to parse (sintaktik xato): {\displaystyle \ce{(RCO)2O + R’OH -> RCO2R' + RCO2H}} </mo></mrow><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{(RCO)2O + R’OH -> RCO2R' + RCO2H}} </mn></mrow></msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{(RCO)2O + R’OH -> RCO2R' + RCO2H}} </mtext><mo> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{(RCO)2O + R’OH -> RCO2R' + RCO2H}} </mo><msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{(RCO)2O + R’OH -> RCO2R' + RCO2H}} </mtext><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{(RCO)2O + R’OH -> RCO2R' + RCO2H}} </mo></msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{(RCO)2O + R’OH -> RCO2R' + RCO2H}} </mtext><mo stretchy="false"> Failed to parse (sintaktik xato): {\displaystyle \ce{(RCO)2O + R’OH -> RCO2R' + RCO2H}} </mo><msubsup><mtext> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{(RCO)2O + R’OH -> RCO2R' + RCO2H}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{(RCO)2O + R’OH -> RCO2R' + RCO2H}} </mn></mrow></msubsup><msup><mtext> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{(RCO)2O + R’OH -> RCO2R' + RCO2H}} </mtext><mo> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{(RCO)2O + R’OH -> RCO2R' + RCO2H}} </mo></msup><mo> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{(RCO)2O + R’OH -> RCO2R' + RCO2H}} </mo><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{(RCO)2O + R’OH -> RCO2R' + RCO2H}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{(RCO)2O + R’OH -> RCO2R' + RCO2H}} </mn></mrow></msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{(RCO)2O + R’OH -> RCO2R' + RCO2H}} </mtext></mrow></mstyle></mrow><annotation encoding="application/x-tex"> </annotation></semantics></math>Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{(RCO)2O + R’OH -> RCO2R' + RCO2H}}
Reaksiyani qaytarib boʻlmaydi va ishni soddalashtiradi. Asilxloridlar va kislota angidridlari ham suv bilan reaksiyaga kirishganligi sababli, suvsiz sharoitlarga afzallik beriladi. Amidlarni hosil qilish uchun aminlarning oʻxshash asilatsiyasi kamroq sezgirdir, chunki aminlar kuchli nukleofillardir va suvga qaraganda tezroq reaksiyaga kirishadi. Faqat laboratoriya miqyosidagi protseduralar uchun qoʻllanadi, chunki u qimmat.
Karboksilat tuzlarining alkillanishi
tahrirEferifikatsiya qilish uchun keng qoʻllanilmasa ham, karboksilat anionlarining tuzlari eferlarni berish uchun alkilgalogenidlar bilan alkillashtiruvchi vosita boʻlishi mumkin. Agar alkilxlorid ishlatilgan boʻlsa, yodid tuzi reaksiyani katalizlashi mumkin (Finkelshteyn reaksiyasi). Karboksilat tuzi koʻpincha joyida hosil boʻladi[12]. Qiyin holatlarda kumush karboksilatdan foydalanish mumkin, chunki kumush ioni galogenid bilan koordinatalanadi, bu uning ketishiga yordam beradi va reaksiya tezligini oshiradi. Ushbu reaksiya anion mavjudligi bilan bogʻliq muammolardan aziyat chekishi mumkin va shuning uchun fazalarni uzatish katalizatorlari yoki DMF kabi yuqori qutbli aprotik erituvchilar qoʻshilishi mumkin.
Transeferifikatsiyasi
tahrirBir eferni boshqasiga almashtirishni oʻz ichiga olgan transeferifikatsiya keng qoʻllanadi.
- <math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow class="MJX-TeXAtom-ORD"><mstyle displaystyle="true" scriptlevel="0"><mrow class="MJX-TeXAtom-ORD"><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{ RCO2R' + CH3OH -> RCO2CH3 + R’OH}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{ RCO2R' + CH3OH -> RCO2CH3 + R’OH}} </mn></mrow></msubsup><msup><mtext> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{ RCO2R' + CH3OH -> RCO2CH3 + R’OH}} </mtext><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{ RCO2R' + CH3OH -> RCO2CH3 + R’OH}} </mo></msup><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{ RCO2R' + CH3OH -> RCO2CH3 + R’OH}} </mo><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{ RCO2R' + CH3OH -> RCO2CH3 + R’OH}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{ RCO2R' + CH3OH -> RCO2CH3 + R’OH}} </mn></mrow></msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{ RCO2R' + CH3OH -> RCO2CH3 + R’OH}} </mtext><mo stretchy="false"> Failed to parse (sintaktik xato): {\displaystyle \ce{ RCO2R' + CH3OH -> RCO2CH3 + R’OH}} </mo><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{ RCO2R' + CH3OH -> RCO2CH3 + R’OH}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{ RCO2R' + CH3OH -> RCO2CH3 + R’OH}} </mn></mrow></msubsup><msubsup><mtext> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{ RCO2R' + CH3OH -> RCO2CH3 + R’OH}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{ RCO2R' + CH3OH -> RCO2CH3 + R’OH}} </mn></mrow></msubsup><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{ RCO2R' + CH3OH -> RCO2CH3 + R’OH}} </mo><msup><mtext> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{ RCO2R' + CH3OH -> RCO2CH3 + R’OH}} </mtext><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{ RCO2R' + CH3OH -> RCO2CH3 + R’OH}} </mo></msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{ RCO2R' + CH3OH -> RCO2CH3 + R’OH}} </mtext></mrow></mstyle></mrow><annotation encoding="application/x-tex"> </annotation></semantics></math>Failed to parse (sintaktik xato): {\displaystyle \ce{ RCO2R' + CH3OH -> RCO2CH3 + R’OH}}
Transeferifikatsiya gidrolizlanish kabi kislotalar va asoslar tomonidan katalizlanadi. Reaksiya triglitseridlarni parchalash uchun keng qoʻllanadi, masalan, yogʻ kislotasi efirlari va spirtli ichimliklarni ishlab chiqarishda. Poli(etilen tereftalat) dimetiltereftalat va etilen glikolning transeferifikatsiyasi natijasida hosil boʻladi.
- <math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow class="MJX-TeXAtom-ORD"><mstyle displaystyle="true" scriptlevel="0"><mrow class="MJX-TeXAtom-ORD"><mrow class="MJX-TeXAtom-ORD"><mo stretchy="false"> </mo><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mo stretchy="false"> </mo></mrow><msubsup><mrow class="MJX-TeXAtom-ORD"><mo stretchy="false"> </mo><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mo stretchy="false"> </mo></mrow><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mo> </mo><mn> </mn><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mrow class="MJX-TeXAtom-ORD"><mo stretchy="false"> </mo><mtext> </mtext><mo stretchy="false"> </mo></mrow><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mo stretchy="false"> </mo><mrow class="MJX-TeXAtom-ORD"><mfrac><mrow><mn> </mn></mrow><mrow class="MJX-TeXAtom-ORD"><mtext class="MJX-tex-mathit" mathvariant="italic"> </mtext></mrow></mfrac></mrow><msubsup><mrow class="MJX-TeXAtom-ORD"><mo stretchy="false"> </mo><mrow class="MJX-TeXAtom-ORD"><mo stretchy="false"> </mo><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mo stretchy="false"> </mo></mrow><msubsup><mrow class="MJX-TeXAtom-ORD"><mo stretchy="false"> </mo><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mo stretchy="false"> </mo></mrow><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mrow class="MJX-TeXAtom-ORD"><mo stretchy="false"> </mo><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mo stretchy="false"> </mo></mrow><mo stretchy="false"> </mo></mrow><mrow class="MJX-TeXAtom-ORD"><mrow class="MJX-TeXAtom-ORD"><mtext class="MJX-tex-mathit" mathvariant="italic"> </mtext></mrow></mrow></msubsup><mo> </mo><mn> </mn><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mtext> </mtext></mrow></mstyle></mrow><annotation encoding="application/x-tex"> </annotation></semantics></math>
Transeferifikatsiyani kichik toʻplami diketenning alkogolizidir. Bu reaksiya, 2-ketoeferlarni beradi.
- <math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow class="MJX-TeXAtom-ORD"><mstyle displaystyle="true" scriptlevel="0"><mrow class="MJX-TeXAtom-ORD"><msubsup><mrow class="MJX-TeXAtom-ORD"><mo stretchy="false"> </mo><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mtext> </mtext><mo stretchy="false"> </mo></mrow><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mo> </mo><mtext> </mtext><mo stretchy="false"> </mo><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mo stretchy="false"> </mo><mtext> </mtext><mo stretchy="false"> </mo></mrow><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mtext> </mtext></mrow></mstyle></mrow><annotation encoding="application/x-tex"> </annotation></semantics></math>
Alkenlar metall karbonil katalizatorlari ishtirokida „gidroeferifikatsiya“ ga uchraydi. Propan kislotasining eferlarini, ushbu usul orqali savdo-sotiq uchun ishlab chiqariladi.
- <math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow class="MJX-TeXAtom-ORD"><mstyle displaystyle="true" scriptlevel="0"><mrow class="MJX-TeXAtom-ORD"><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mo> </mo><mtext> </mtext><mo> </mo><mtext> </mtext><mo stretchy="false"> </mo><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mtext> </mtext></mrow></mstyle></mrow><annotation encoding="application/x-tex"> </annotation></semantics></math>
Metil propionat preparati yorqin misoldir.
- <math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow class="MJX-TeXAtom-ORD"><mstyle displaystyle="true" scriptlevel="0"><mrow class="MJX-TeXAtom-ORD"><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mo> </mo><mtext> </mtext><mo> </mo><mtext> </mtext><mo stretchy="false"> </mo><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup></mrow></mstyle></mrow></semantics></math>
Metanolning karbonillanishi formik kislotaning asosiy tijorat manbai boʻlgan metil formatini hosil qiladi. Reaksiya natriy metoksid bilan katalizlanadi.
- <math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow class="MJX-TeXAtom-ORD"><mstyle displaystyle="true" scriptlevel="0"><mrow class="MJX-TeXAtom-ORD"><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mtext> </mtext><mo> </mo><mtext> </mtext><mo stretchy="false"> </mo><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mtext> </mtext></mrow></mstyle></mrow><annotation encoding="application/x-tex"> </annotation></semantics></math>
Karbon kislotalarning alken va alkinlarga qoʻshilishi
tahrirGidroeferifikatsiyada alkenlar va alkinlar karboksilik kislotalarning H-O bogʻiga qoʻshiladi. Vinil asetat sanoatda rux asetat katalizatorlari ishtirokida asetilenga sirka kislota qoʻshilishi yoʻli bilan ishlab chiqariladi. Hozirda katalizator sifatida rux asetat qoʻllanadi.
- <math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow class="MJX-TeXAtom-ORD"><mstyle displaystyle="true" scriptlevel="0"><mrow class="MJX-TeXAtom-ORD"><msubsup><mtext> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mn></mrow></msubsup><msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mtext><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mo></msup><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mo><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mn><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mn></mrow></msubsup><mo stretchy="false"> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mo><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mn></mrow></msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mtext><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mo><msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mtext><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mo></msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mtext></mrow></mstyle></mrow><annotation encoding="application/x-tex"> </annotation></semantics></math>Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}}
Vinil asetat, etilen, sirka kislotasi va kislorodning, palladiy bilan katalizlangan reaksiyasi orqali ham ishlab chiqarish mumkin.
- Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}}
Silikotungstik kislotasi, etilen bilan sirka kislotasini, alkillash orqali etil asetat ishlab chiqarish uchun ishlatiladi.
- <math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow class="MJX-TeXAtom-ORD"><mstyle displaystyle="true" scriptlevel="0"><mrow class="MJX-TeXAtom-ORD"><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mo> </mo><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><mtext> </mtext><mo stretchy="false"> </mo><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup><msubsup><mtext> </mtext><mrow class="MJX-TeXAtom-ORD"><mn> </mn></mrow></msubsup></mrow></mstyle></mrow></semantics></math> </img>
Aldegidlarda
tahrirTishchenko reaksiyasi suvsiz asos ishtirokida aldegidni disproporsiyalash orqali efir hosil qiladi. Katalizatorlar sifatida alyuminiy alkoksidlari yoki natriy alkoksidlari ishlatiladi. Benzaldegid natriy benziloksid (natriy va benzil spirtidan hosil boʻlgan) bilan reaksiyaga kirishib, benzil benzoat hosil qiladi. Bu usul orqali asetaldegiddan, etil asetat ishlab chiqarishda qoʻllanadi.
Boshqa usullari
tahrir- Baza borligida α- galoketonlarning Favorskiy qayta joylash
- Beyer-Villiger ketonlarning peroksidlar bilan oksidlanishi
- Nitrillarning alkogol bilan pinner reaksiyasi
- Metall-atsil kompleksining nukleofil abstraktsiyasi
- Ortoeferlarning suvli kislotada gidrolizlanishi
- Eferifikatsiya orqali Sellyuloliz[13]
- Xlorid kislota va turli spirtlar ishtirokida ishlaydigan alkenlarning ozonolizi[14].
- Metil eferlarga olib keladigan metil ketonlarning anodik oksidlanishi[15].
- Intereferifikatsiya turli efirlarning yog 'kislotalari guruhlarini almashtiradi.
Reaksiyalari
tahrirEferlar, uglerod karbonili orqali nukleofillar bilan reaksiyaga kirishadi. Karbonil zaif elektrofil, ammo kuchli nukleofillar (aminlar, alkoksidlar, gidrid manbalari, organolitiy birikmalari va boshqalar) bilan reaksiyaga kirishadi. Karbonilga tutashgan C–H aloqalari zaif kislotali, ammo kuchli asoslar bilan deprotonatsiyaga uchraydi. Bu jarayon odatda kondensatsiya reaksiyalarini boshlaydi. Efirlardagi karbonil kislorod, amidlardagi azotdan elektron juftining rezonansli donatsiyasi tufayli, amidlardagi karbonil kisloroddan kamroq qo'shimchalar hosil qiladi.
Gidroliz va sovunlanishi
tahrirEferifikatsiya teskari reaksiyadir. Eferlar kislotali va asosli sharoitlarda gidrolizga uchraydi. Kislotali sharoitda reaksiya Fisher esterifikatsiyasining teskari reaksiyasi hisoblanadi. Asosiy sharoitlarda gidroksid nukleofil rolini oʻynaydi, alkoksid esa tark etuvchi guruhdir. Bu reaksiya sovunlanish, sovun tayyorlashning asosidir.
Alkoksid guruhi kuchliroq nukleofillar, masalan, ammiak birlamchi yoki ikkilamchi aminlar bilan almashtirilib, amidlarni hosil qilishi mumkin.
- <math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow class="MJX-TeXAtom-ORD"><mstyle displaystyle="true" scriptlevel="0"><mrow class="MJX-TeXAtom-ORD"><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + NH2R'' -> RCONHR'' + R’OH}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + NH2R'' -> RCONHR'' + R’OH}} </mn></mrow></msubsup><msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + NH2R'' -> RCONHR'' + R’OH}} </mtext><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + NH2R'' -> RCONHR'' + R’OH}} </mo></msup><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + NH2R'' -> RCONHR'' + R’OH}} </mo><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + NH2R'' -> RCONHR'' + R’OH}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + NH2R'' -> RCONHR'' + R’OH}} </mn></mrow></msubsup><msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + NH2R'' -> RCONHR'' + R’OH}} </mtext><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + NH2R'' -> RCONHR'' + R’OH}} </mo></msup><mo stretchy="false"> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + NH2R'' -> RCONHR'' + R’OH}} </mo><msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + NH2R'' -> RCONHR'' + R’OH}} </mtext><mo> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2R' + NH2R'' -> RCONHR'' + R’OH}} </mo></msup><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + NH2R'' -> RCONHR'' + R’OH}} </mo><msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + NH2R'' -> RCONHR'' + R’OH}} </mtext><mo> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2R' + NH2R'' -> RCONHR'' + R’OH}} </mo></msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + NH2R'' -> RCONHR'' + R’OH}} </mtext></mrow></mstyle></mrow><annotation encoding="application/x-tex"> </annotation></semantics></math>Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2R' + NH2R'' -> RCONHR'' + R’OH}}
Bu reaksiya odatda qaytmaydi. Aminlar oʻrniga gidrazinlar va gidroksilaminlar qoʻllanishi mumkin. Eferlar Lossen qayta tashkil etishda oraliq gidroksamik kislotalar orqali izosiyanatlara aylantirilishi mumkin.
Uglerod nukleofillarining manbalari, Grignard reagentlari, organolitiy birikmalari karbonilga osongina qoʻshiladi.
Kamaytirilishi
tahrirKetonlar va aldegidlar bilan solishtirganda, efirlar qaytarilishga nisbatan chidamli. XX-asrning boshlarida katalitik gidrogenatsiyaning kiritilishi yutuq boʻldi. Yogʻ kislotalarining eferlari yog'li spirtlarga vodorodlanadi.
- <math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow class="MJX-TeXAtom-ORD"><mstyle displaystyle="true" scriptlevel="0"><mrow class="MJX-TeXAtom-ORD"><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mn></mrow></msubsup><msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mtext><mo> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mo></msup><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mo><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mn><msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mn></mrow></msubsup><mo stretchy="false"> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mo><msubsup><mtext> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mtext><mrow class="MJX-TeXAtom-ORD"><mn> Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "http://localhost:6011/uz.wikipedia.org/v1/":): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mn></mrow></msubsup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mtext><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mo><msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mtext><mo> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mo></msup><mtext> Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}} </mtext></mrow></mstyle></mrow><annotation encoding="application/x-tex"> </annotation></semantics></math>Failed to parse (sintaktik xato): {\displaystyle \ce{RCO2R' + 2 H2 -> RCH2OH + R’OH}}
Odatda katalizator sifatida mis xromitdan foydalanilgan. Katalitik gidrogenatsiyani ishlab chiqishdan oldin efirlar Bouveauult-Blanc reduksiyasi yordamida keng miqyosda kamaytirilgan. Koʻp jihatdan eskirgan bu usul proton manbalari mavjudligida natriydan foydalanilgan.
Ayniqsa, nozik kimyoviy sintezlar uchun lityum alyuminiy gidrid eferlarni ikkita asosiy spirtga kamaytirish uchun ishlatiladi. Tegishli reagent natriy borgidrid bu reaksiyada sekin. DIBAH eferlarni aldegidlarga kamaytiradi[16].
Tegishli efirni berish uchun toʻgʻridan-toʻgʻri qaytarilish qiyin, chunki oraliq yarimatsetal parchalanishga moyil boʻlib, spirt va aldegidni beradi. Reaksiyani turli Lyuis kislotalari bilan trietilsilan yordamida amalga oshirish mumkin[17][18].
Boshqa reaksiyalari
tahrir- Fenil eferlar Fries qayta tashkil etishda gidroksiarilketonlar bilan reaksiyaga kirishadi.
- Maxsus efirlar Chan qayta tashkil etilishida α-gidroksil guruhi bilan ishlaydi.
- β-vodorod atomlari boʻlgan eferlar, ester pirolizida alkenlarga aylanishi mumkin.
- Esterlarning toʻgʻridan-toʻgʻri nitrillarga aylanishi[19].
Eferlar sinfi karboksilik kislotalar uchun himoya guruhlari boʻlib xizmat qiladi. Karboksilik kislotani himoya qilish bifunksional aminokislotalarning reaksiyasini oldini olish uchun peptid sintezida foydalidir. Koʻpgina aminokislotalar uchun metil va etil eferlar odatda mavjud. t-butil efer qimmat boʻladi. Shu bilan birga, t-butil efirlari foydali, chunki kuchli kislotali sharoitda t -butil efirlari eliminatsiyadan oʻtib, karboksilik kislota va izobutilen hosil qiladi, bu esa ishni soddalashtiradi.
Efer hidli moddalar roʻyxati
tahrirKoʻpgina eferlar mevaga oʻxshash oʻziga xos hidlarga ega va ularning koʻplari tabiiy ravishda oʻsimliklarning efir moylarida uchraydi. Shuningdek, ularni sunʼiy xushboʻy hidlarni taqlid qilishda keng qoʻllanadi.
| Efer nomi | Tuzilishi | Hid yoki hodisasi |
|---|---|---|
| Allil geksanoat | ananas | |
| Benzil asetat | nok, qulupnay, yasmin | |
| Bornil asetat | qaragʻay | |
| Butil asetat | olma, asal | |
| Butil butirat | ananas | |
| Butil propanoat | nok tomchilari | |
| Etil asetat | tirnoq boʻyogʻini tozalash vositasi, model boʻyoq, model samolyot yelimi, nok | |
| Etil benzoat | shirin, yashil, mevali, dorivor, olcha, uzum | |
| Etil butirat | banan, ananas, qulupnay | |
| Etil geksanoat | ananas, mumsimon yashil banan | |
| Etil sinnamat | dolchin | |
| Ethyl formate | limon, rom, qulupnay | |
| Etil heptanoat | oʻrik, olcha, uzum, malina | |
| Etil izovalerat | olma | |
| Etil laktat | sariyogʻ , qaymoq | |
| Etil nonanoat | uzum | |
| Etil pentanoat | olma | |
| Geranil asetat | geranium | |
| Geranyl butyrate | gilos | |
| Geranil pentanoat | olma | |
| Izobutil asetat | gilos, malina, qulupnay | |
| Izobutil formati | malina | |
| Izoamil asetat | nok, banan (nok tomchilarida lazzatlanish) | |
| Izopropil asetat | mevali | |
| Linalil asetat | lavanta | |
| Linalyl butyrate | shaftoli | |
| Linalyl formate | apple, peach | |
| Methyl acetate | glue | |
| Methyl anthranilate | grape, jasmine | |
| Methyl benzoate | fruity, ylang ylang, feijoa | |
| Methyl butyrate (methyl butanoate) | pineapple, apple, strawberry | |
| Methyl cinnamate | strawberry | |
| Methyl pentanoate (methyl valerate) | flowery | |
| Methyl phenylacetate | honey | |
| Methyl salicylate (oil of wintergreen) | Modern root beer, wintergreen, Germolene and Ralgex ointments (UK) | |
| Nonil kaprilat | apelsin | |
| Octyl acetate | fruity-orange | |
| Octyl butyrate | parsnip | |
| Amil asetat (pentil asetat) | olma, banan | |
| Pentyl butyrate (amyl butyrate) | apricot, pear, pineapple | |
| Pentyl hexanoate (amyl caproate) | apple, pineapple | |
| Pentyl pentanoate (amyl valerate) | olma | |
| Propyl acetate | pear | |
| Propyl hexanoate | blackberry, pineapple, cheese, wine | |
| Propyl isobutyrate | rum | |
| Terpenyl butyrate | cherry |
Yana qarang
tahrir- Esterlar ro'yxati
- Amid, azot bilan almashtirilgan kislorodli ester analogi
- Siyanat esteri
- Oligoester
- Poliester
- Tioester, oltingugurt bilan almashtirilgan kislorodli ester analogi
- Transesterifikatsiya
- Efir lipidlari
Manbalar
tahrir- ↑ Cameron Wright. A worker's guide to solvent hazards. The Group, 1986 — 48-bet. ISBN 9780969054542.
- ↑ E. Richard Booser. CRC Handbook of Lubrication and Tribology, Volume III: Monitoring, Materials, Synthetic Lubricants, and Applications. CRC, 21 December 1993 — 237-bet. ISBN 978-1-4200-5045-5.
- ↑ Leopold Gmelin, Handbuch der Chemie, vol. 4: Handbuch der organischen Chemie (vol. 1) (Heidelberg, Baden (Germany): Karl Winter, 1848), page 182.
- ↑ 4,0 4,1 March, J. Advanced Organic Chemistry 4th Ed. J. Wiley and Sons, 1992: New York. ISBN 0-471-60180-2
- ↑ „Chemistry of Enols and Enolates – Acidity of alpha-hydrogens“.
- ↑ Diwakar M. Pawar; Abdelnaser A. Khalil; Denise R. Hooks; Kenneth Collins; Tijuana Elliott; Jefforey Stafford; Lucille Smith; Eric A. Noe (1998). „E and Z Conformations of Esters, Thiol Esters, and Amides“. J. Am. Chem. Soc. 120-jild, № 9. 2108–2112-bet. doi:10.1021/ja9723848.
- ↑ Christophe Dugave; Luc Demange (2003). „Cis−Trans Isomerization of Organic Molecules and Biomolecules: Implications and Applications“. Chem. Rev. 103-jild, № 7. 2475–2932-bet. doi:10.1021/cr0104375. PMID 12848578.
- ↑ A. A. Yakovenko, J. H. Gallegos, M. Yu. Antipin, A. Masunov, T. V. Timofeeva (2011). „Crystal Morphology as an Evidence of Supramolecular Organization in Adducts of 1,2-Bis(chloromercurio)tetrafluorobenzene with Organic Esters“. Cryst. Growth Des. 11-jild, № 9. 3964–3978-bet. doi:10.1021/cg200547k.
{{cite magazine}}: CS1 maint: multiple names: authors list () - ↑ Isolation of triglyceride from nutmeg: G. D. Beal „Trimyristen“ Organic Syntheses, Coll.
- ↑ McGee, Harold.
- ↑ Williams, Roger J.; Gabriel, Alton; Andrews, Roy C. (1928). „The Relation Between the Hydrolysis Equilibrium Constant of Esters and the Strengths of the Corresponding Acids“. J. Am. Chem. Soc. 50-jild, № 5. 1267–1271-bet. doi:10.1021/ja01392a005.
- ↑ Matsumoto, Kouichi; Shimazaki, Hayato; Miyamoto, Yu; Shimada, Kazuaki; Haga, Fumi; Yamada, Yuki; Miyazawa, Hirotsugu; Nishiwaki, Keiji; Kashimura, Shigenori (2014). „Simple and Convenient Synthesis of Esters from Carboxylic Acids and Alkyl Halides Using Tetrabutylammonium Fluoride“. Journal of Oleo Science (inglizcha). 63-jild, № 5. 539–544-bet. doi:10.5650/jos.ess13199. ISSN 1345-8957. PMID 24770480.
- ↑ Ignatyev, Igor; Charlie Van Doorslaer; Pascal G.N. Mertens; Koen Binnemans; Dirk. E. de Vos (2011). „Synthesis of glucose esters from cellulose in ionic liquids“. Holzforschung. 66-jild, № 4. 417–425-bet. doi:10.1515/hf.2011.161.
- ↑ Neumeister, Joachim; Keul, Helmut; Pratap Saxena, Mahendra; Griesbaum, Karl (1978). „Ozone Cleavage of Olefins with Formation of Ester Fragments“. Angewandte Chemie International Edition in English. 17-jild, № 12. 939–940-bet. doi:10.1002/anie.197809392.
- ↑ Makhova, Irina V.; Elinson, Michail N.; Nikishin, Gennady I. (1991). „Electrochemical oxidation of ketones in methanol in the presence of alkali metal bromides“. Tetrahedron. 47-jild, № 4–5. 895–905-bet. doi:10.1016/S0040-4020(01)87078-2.
- ↑ W. Reusch. „Carboxyl Derivative Reactivity“. Virtual Textbook of Organic Chemistry. 2016-yil 16-mayda asl nusxadan arxivlangan.
- ↑ Yato, Michihisa; Homma, Koichi; Ishida, Akihiko (June 2001). „Reduction of carboxylic esters to ethers with triethyl silane in the combined use of titanium tetrachloride and trimethylsilyl trifluoromethanesulfonate“. Tetrahedron. 57-jild, № 25. 5353–5359-bet. doi:10.1016/S0040-4020(01)00420-3.
- ↑ Sakai, Norio; Moriya, Toshimitsu; Konakahara, Takeo (July 2007). „An Efficient One-Pot Synthesis of Unsymmetrical Ethers: A Directly Reductive Deoxygenation of Esters Using an InBr3/Et3SiH Catalytic System“. The Journal of Organic Chemistry. 72-jild, № 15. 5920–5922-bet. doi:10.1021/jo070814z. PMID 17602594.
- ↑ Wood, J. L.; Khatri, N. A.; Weinreb, S. M. (1979). „A direct conversion of esters to nitriles“. Tetrahedron Letters. 20-jild, № 51. 4907-bet. doi:10.1016/S0040-4039(01)86746-0.